Metals are extensively used in
art and industry.
Iron in the form of steel
wrought iron and cast iron is used for the manufacturing of articles of
locomotion, railways watch strings, cutlery, sanitary fitting tubes, sheets and
bars etc.
Copper is used in electrical
industries and for making various kinds of alloys.
Aluminum is used in the
manufacturing of coins.
Zinc and tin are used to coat
iron to prevent it from rusting.
1. Physical Properties Of
Metals and Non-Metals:
1.1. Physical Properties Of Metals:
They posses luster which is
known as metallic luster.
They are good conductor of
electricity.
They are electro-positive.
They are malleable and
ductile.
They have high specific
gravity.
The reflect light.
They produce metallic sound
upon strike.
They are generally solid.
1.1.1. Examples:
Aluminum, Copper. Iron.
1.2. Physical Properties
Of Non-Metals:
They does not produce metallic
luster.
They are bad conductor of
electricity.
They are bad conductor of
heat.
They are electronegative.
They cannot be drawn into
sheets and wires.
They have low specific
gravity.
They does not reflect light.
They does not produce metallic
sound.
They are found in all states
of matter.
1.2.1. Example:
Oxygen, bromide, sulphur.
2. Chemical Properties Of
Metals and Non Metals.
2.1. Chemical Properties Between Metals:
Metallic oxides are basic in
nature.
They frequently dissolve in
dilute mineral acid to liberate hydrogen gas.
They either do not combine
with hydrogen and form non volatile hydrides.
Their chlorides are not
hydrolyzed by water.
2.2. Non Metals:
Non metallic oxides are acidic
in nature.
They does not dissolve in
dilute mineral acids.
They form stable compound with
hydrogen.
Their chlorides are completely
hydrolyzed by water.
PHYSICAL PROPERTIES OF NON-METALS
Non-metals are Non-malleable
or Brittle:- Non-metals break into pieces when stretched. For example, sulphur and phosphorous are brittle non-metals
Non-metals are Non-ductile:-
Non-metals cannot be drawn into thin wires i. e. they broke into pieces when
stretched
Non-metals are Bad Conductors
of Heat and Electricity:- Generally Non-metals are bad conductors of heat and
electricity because they do not have free electrons responsible for transfer of
heat and electricity
Non-metals are non-lustrous
and cannot be polished
Non-metals may be solid,
liquid or Gases at room temperature
Non-metals have low melting
and boiling points as compared to metals
Most of solid metals are soft
Non-metals are not strong
Non-metals are light
substances
Non-metals are non-sonorous
CHEMICAL PROPERTIES OF
NON-METALS
Non-metals
are also called electronegative elements because the non-metal atom form
negatively charged ion by accepting electrons. Following are the important chemical
reactions of non-metals.
1. Reaction of Non-metals with Oxygen
All non-metals react with oxygen to form acidic or neutral oxides. For example, Carbon forms acidic carbon dioxide on reacting with oxygen.
1. Reaction of Non-metals with Oxygen
All non-metals react with oxygen to form acidic or neutral oxides. For example, Carbon forms acidic carbon dioxide on reacting with oxygen.
C +
O2  CO2
CO2
Carbon Carbon Dioxide
Carbon Carbon Dioxide
2. Reaction of Non-metals with
Water
Non-metals do not react with water.
3. Reaction of Non-metals with Dilute Acids
Non-metals do not react with dilute acids and don’t displace hydrogen from dilute acids, because non-metals are electron acceptor. So, they cannot supply electrons to H+ ions. So, they do not displace hydrogen from dilute acids.
4. Reaction of Non-metals with Chlorine
Non-metals react with chlorine to form covalent chlorides. For example,
Non-metals do not react with water.
3. Reaction of Non-metals with Dilute Acids
Non-metals do not react with dilute acids and don’t displace hydrogen from dilute acids, because non-metals are electron acceptor. So, they cannot supply electrons to H+ ions. So, they do not displace hydrogen from dilute acids.
4. Reaction of Non-metals with Chlorine
Non-metals react with chlorine to form covalent chlorides. For example,
H2
+ Cl2
 2HCl
2HCl
Hydrogen Chlorine Hydrogen chloride
5. Reaction of Non-metals with Hydrogen
Non-metals react with hydrogen to form covalent Hydrides. For example,
Hydrogen Chlorine Hydrogen chloride
5. Reaction of Non-metals with Hydrogen
Non-metals react with hydrogen to form covalent Hydrides. For example,
H2
+ S  H2S
H2S
Hydrogen sulphur Hydrogen sulphide
Hydrogen sulphur Hydrogen sulphide
DIFFERENCES BETWEEN PROPERTIES
OF METALS AND NON-METALS
Differences in Physical Properties
|
Metals
|
Non-Metals
|
|
Metals are malleable.
|
Non-metals
are non-malleable or brittle.
|
|
Metals are ductile.
|
Non-metals
are non-ductile
|
|
Metals
are good conductors of heat and electricity.
|
Non-metals
are bad conductors of heat and electricity.
|
|
Metals
are Lustrous and can be polished.
|
Non-metals
are non-lustrous and cannot be polished.
|
|
Metals
are solid at room temperature.
|
Non-metals
may be solid, liquid or Gases at room temperature.
|
|
The
melting and boiling points of metals are generally high.
|
Non-metals
have low melting and boiling points as compared to metals.
|
|
All metals are strong.
|
Non-metals
are not strong.
|
|
Generally, metals are hard.
|
Most
of Non-metals are soft.
|
|
Metals are heavy.
|
Non-metals
are light substances.
|
|
Metals are sonorous.
|
Non-metals
are non-sonorous.
|
Differences in Chemical Properties
|
Metals
|
Non-Metals
|
|
All
metals react with oxygen to form metal oxides
|
All
non-metals react with oxygen to form acidic or neutral oxides.
|
|
Metals
react with water to produce metal oxide (or metal hydroxide) and hydrogen
gas.
|
Non-metals
do not react with water.
|
|
Metals
react with a dilute acid to form a metal salt and hydrogen gas.
|
Non-metals
do not react with dilute acids .
|
|
All
metals react with chlorine to form ionic metal chlorides.
|
Non-metals
react with chlorine to form covalent chlorides.
|
|
Only
a few metals like Na, K, Ca and Mg react with hydrogen to form metal
hydrides.
|
Non-metals
react with hydrogen to form covalent Hydrides.
|
Iron
Physical Properties: ductile,
malleable, silver-gray metal, lustrous, and shiny
Chemical Properties: flakes off when in water
Chemical Properties: flakes off when in water
Gold
Physical Properties: yellow,
metallic, shiny, rough,malleable
Chemical Properties: when divided, its black
Chemical Properties: when divided, its black
Silver
Physical Properties: shiny,
gray, smooth, metallic, malleable
Chemical Properties: precipitates into a white color
Chemical Properties: precipitates into a white color
Oxygen
Physical Properties:
colorless, odorless, tasteless, can’t see it, you can breath it
Chemical Properties: reacts with iron to form rust
Chemical Properties: reacts with iron to form rust
Nitrogen
Physical Properties:
colorless, odorless, tasteless, can see it, not able to breath
Chemical Properties: can fuse with hydrogen to form ammonia
Chemical Properties: can fuse with hydrogen to form ammonia
Sulfur
Physical Properties: abundant,
tasteless and odorless, can see it, can't breath it
Chemical Properties: does not react with water under normal conditions
Chemical Properties: does not react with water under normal conditions


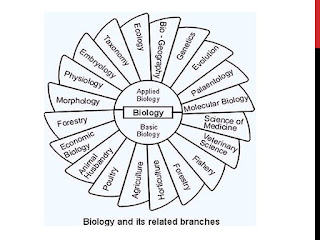
Cytology
|
It is the branch of biology which deals with
the study of descent of present day complex living organims (plants and
animals) from the living forms of the past.
|
Biotechnology
|
|
Ecology
|
Study of bacteria.
|
Physiology
|
It is the study of inter—relationship between
living organisms and their environment.
|
Molecular biology
|
Study of functions of various parts of body
is called physiology. |
Histology
|
It deals with the study of the stages of an
organism that occur immediately after fertilization.
|
Embryology
|
Study of chemical aspect of living organims is
termed biochemistry.
|
Virology
|
Study of living organisms at the molecular
level.
|
Genetics
|
It deals with the study of structure and
functions of cell.
|
It is the study of viruses.
|
|
Mycology
|
The study of the structure and chemical
composition of animal and plant tissue as related to the function.
|
Bacteriology
|
It is the controlled use of biological agents
such as micro-organisms or cellular components for beneficial use.
|
Anatomy
|
It is a
branch of biology which deals with the study of internal structure of
an organism as revealed by dissection.
|
Biochemistry
|
It is the study of fungi.
|

Inorganic chemistry is the study of the
synthesis, reactions, structures and properties of compounds of the elements.
This subject is usually taught after students are introduced to organic
chemistry, which concerns the synthesis and reactions of compounds of carbon
(typically containing C-H bonds). Inorganic chemistry encompasses the compounds
- both molecular and extended solids - of everything else in the periodic table, and overlaps with organic
chemistry in the area of organometallic chemistry, in which metals are bonded
to carbon-contaning ligands and molecules. Inorganic chemistry is fundamental
to many practical technologies including catalysis and materials (structural,
electronic, magnetic,...), energy conversion and storage, and electronics.
Inorganic compounds are also found in biological systems where they are
essential to life processes.
Chemical Bonding
Molecules are built from atoms
that form chemical bonds. Theories of bonding seek to explain why molecules and
solids form, what their structures are, why some are more stable than others,
and how they react.
The theory of chemical bonding
has a long history, dating back to ancient Greece and the atomists Democritus, Leucippus, and the Epicureans. The Roman poet Lucretius (c. 99 BC – c. 50 BC),
drawing on his Epicurean beliefs, describes some atoms and chemical bonding in
the following way:
What seems to us the hardened
and condensed
Must be of atoms among themselves more hooked,
Be held compacted deep within, as 'twere
By branch-like atoms -- of which sort the chief
Are diamond stones, despisers of all blows
And stalwart flint and strength of solid iron
And brazen bars, which, budging hard in locks,
Do grate and scream. But what are liquid, formed
Of fluid body, they indeed must be
Of elements more smooth and round -- because
Their globules severally will not cohere.
Must be of atoms among themselves more hooked,
Be held compacted deep within, as 'twere
By branch-like atoms -- of which sort the chief
Are diamond stones, despisers of all blows
And stalwart flint and strength of solid iron
And brazen bars, which, budging hard in locks,
Do grate and scream. But what are liquid, formed
Of fluid body, they indeed must be
Of elements more smooth and round -- because
Their globules severally will not cohere.
Lucretius' poem is enjoyable
reading and contains some remarkable insights into the microscopic world, given
the tools available at the time. Modern analytical methods show that he was off
base with his ideas about hooks and spheres, however. We will revisit the
nature of chemical bonding in the substances Lucretius mentions (diamond,
silicates, iron, brass, and water) in the context of modern chemical theories
to understand why they have the special properties they do.
Basic elements and their roles
Elements are basic ingredients of the animate
and inanimate world. Nitrogen (N), sulphur (S), oxygen (O), hydrogen (H), and
carbon (C) form organic compounds, i.e. proteins, carbohydrates, fats, and
vitamins.
App. 1/3 of 104 known elements are components
important for organisms, i.e. structural elements of skeleton and soft tissues,
and factors regulating many physiological functions, e.g. blood coagulation,
oxygen transport, and enzyme activation.
Groups of elements
These elements can be divided into three groups:
elements necessary for life, i.e. bioelements
neutral elements without which metabolic
processes can proceed normally
toxic elements with adverse effects on organisms
Macro- and microelements
Elements necessary for the correct functioning
of the body are classified as macro- or microelements.
The levels of macroelements in bodily fluids and
tissues exceed 1 μg/g of wet tissue (μg – one millionth part of a gram – 10-6 g) Macroelements
include:
chlorine
(Cl) phosphorus (P) magnesium (Mg) potassium (K)
sodium
(Na) calcium (Ca)
The levels of microelements (trace elements) in
the organism are below 1 μg/g of wet tissue. Microelements include:
germanium
(Ge) boron (B) chromium (Cr) tin
(Sn) zinc (Zn)
fluorine
(F) iodine (I) cobalt (Co) silicon (Si) lithium (Li)
manganese
(Mn) copper (Cu) molybdenum (Mo) nickel
(Ni)
selenium
(Se) vanadium (V) iron (Fe)
Toxic elements include:
aluminium
(Al) thallium (Tl) mercury (Hg) cadmium (Cd) lead
(Pb)
Toxicity of chemical elements depends on many
factors; the most important of them are the levels of an element in the body
and the time of exposure. A significant role is played by the ability of the
body to eliminate harmful elements; such functions are performed by kidneys,
the liver, and the digestive system. Harmful influence of toxic elements
depends on the abilities of the organism to repair their adverse effects.
Toxic elements tend to accumulate in
parenchymatous organs, in particular in the liver, kidneys, and the pancreas.
During a prolonged exposure, toxic elements can
also accumulate in other tissues, e.g. lead and aluminium in bones, lead
mercury, and aluminium in brain tissues, and cadmium in hair roots.
Physiological life processes depend not only on
the composition and concentration of separate elements but also on their
proportions in the body. For separate areas of the body, there is a precisely
specified ion balance, which is maintained stable. Based on proportions between
certain elements, metabolic activity may be assessed as well as the correctness
of physiological processes. There are synergistic and antagonistic
relationships between trace elements which directly influence metabolism in the
body.
In many cases, maintaining correct relationships
between separate elements is more important than their correct concentration.
Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N),
Sulphur (S), Phosphorus (P), Potassium (K) , Calcium(Ca), Magnesium(Mg), Iron
(Fe), Boron(B), Maganaese (Mn), Zinc(Zn), Copper (Cu), Molybdenum (Mo) and
Chlorine (Cl) are required for the growth and development of plants. These
elements are known as essential elements. They are divided into two
groups depending upon their requirement.
a) Macroelements (Macronutrients or Major elements):
Those element which are required in large quantities are called
macronutrients. They are usually participate in body construction
and are ten in number.
b) Microelements (Micronutrients or Minor element or
Trace element): Those element which are required in small quantities are called
microelement or trace element. They are usually participate in in various
metabolisms and are six in number.
Microelements
vs Macroelements
Microelements
(Micronutrients)
|
Macroelements
(Macronutrients)
|
They occur in
plants in very small amounts.
|
They occur in
plants in easily detectable quantities.
|
The
concentration of a microelement is less than 1 mg/gm of dry matter.
|
The
concentration of a macroelement per gram of dry matter is at least 1 mg or
1000 microgram.
|
Microelements
do not have such a role.
|
They build up
the plant body and different protoplasmic constituents.
|
Microelements,
being found in traces only, have no significant role in the development of
osmotic potential.
|
Some
macroelements accumulate in cell sp and take part in developing osmotic
potential.
|
None of the
microelements have any such function.
|
Turgor
movements are mostly caused by influx and efflux of potassium, a
macroelement.
|
Microelement
are toxic in slight excess.
|
They do not
become toxic in slight excess.
|
Microelements
are : Zn, Mn, B, Cu, Mo, Cl, and Ni.
|
Macroelements
are: C, H, O, N, P, K, S, Mg, Fe and Ca.
|
Classification of compounds
Chemical compounds may be classified according
to several different criteria. One common method is based on the specific
elements present. For example, oxides contain one or more oxygen
atoms, hydrides contain one or more hydrogen
atoms, and halides contain one or more halogen (Group 17) atoms. Organic compounds are characterized as those
compounds with a backbone of carbon atoms, and all the remaining compounds are
classified as inorganic. As the name suggests, organometallic compounds are organic compounds bonded
to metal atoms.
Another classification scheme for chemical
compounds is based on the types of bonds that the compound contains. Ionic compounds contain ions and are held
together by the attractive forces among the oppositely charged ions. Common salt (sodium chloride) is one of
the best-known ionic compounds. Molecular compounds contain discrete molecules, which are held together by
sharing electrons (covalent bonding). Examples are water, which contains H2O
molecules; methane, which contains CH4
molecules; and hydrogen fluoride, which contains HF molecules.
A third classification scheme is based on
reactivity—specifically, the types of chemical reactions that the compounds are likely
to undergo. For example, acids are compounds that produce H+
ions (protons) when dissolved in water to
produce aqueous solutions. Thus, acids are defined as proton
donors. The most common acids are aqueous solutions of HCl (hydrochloric acid), H2SO4
(sulfuric acid), HNO3 (nitric acid), and H3PO4
(phosphoric acid). Bases, on the other hand, are proton acceptors. The most common base is the hydroxide ion (OH−), which
reacts with an H+ ion to form a water molecule.H+ + OH−
→ HOH (usually written H2O)
Oxidation-reduction
reactions
constitute another important class of chemical reactions. Oxidation involves a
loss of electrons, whereas reduction involves a gain of electrons. For example,
in the reaction between sodium metal and chlorine gas to form sodium chloride,2Na + Cl2 → 2NaCl,
electrons (e−) are transferred from sodium atoms to chlorine atoms
to form Na+ and Cl− ions in the reaction product, sodium
chloride.
In this process, each sodium atom loses an
electron and is thus oxidized, and each chlorine atom gains an electron and is
thus reduced. In this reaction, sodium is called the reducing agent (it furnishes electrons), and
chlorine is called the oxidizing agent (it consumes electrons). The
most common reducing agents are metals, for they tend to lose electrons in
their reactions with nonmetals. The most common oxidizing agents are
halogens—such as fluorine (F2), chlorine (Cl2), and
bromine (Br2)—and certain oxy anions, such as the permanganate ion
(MnO4−) and the dichromate ion (Cr2O72−).
Inorganic compounds include compounds that are
made up of two or more elements other than carbon, as well as certain
carbon-containing compounds that lack carbon-carbon bonds, such as cyanides and carbonates. Inorganic compounds are most
often classified in terms of the elements or groups of elements that they
contain. Oxides, for example, can be either
ionic or molecular. Ionic oxides contain O2− (oxide) ions and metal cations, whereas molecular oxides
contain molecules in which oxygen (O) is covalently bonded to
other nonmetals such as sulfur (S) or nitrogen (N). When ionic oxides are
dissolved in water, the O2− ions
react with water molecules to form hydroxide ions (OH−), and a
basic solution results. Molecular oxides react with water to produce oxyacids, such as sulfuric acid (H2SO4)
and nitric acid (HNO3). In
addition, inorganic compounds include hydrides (containing hydrogen atoms or
H− ions), nitrides (containing N3−
ions), phosphides (containing P3−
ions), and sulfides (containing S2−
ions).
Borane is an example of an inorganic compound.
Transition metals form a great variety of
inorganic compounds. The most important of these are coordination compounds in which the metal atom or
ion is surrounded by two to six ligands. Ligands are ions or neutral
molecules with electron pairs that they can donate to
the metal atom to form a coordinate-covalent bond.
The resulting covalent bond is given a special name
because one entity (the ligand) furnishes both of the electrons that are
subsequently shared in the bond. An example of a coordination
compound is [Co(NH3)6]Cl3,
which contains the Co(NH3)63+ ion, a cobalt ion (Co3+) with
six ammonia molecules (NH3)
attached to it, acting as ligands.
In the early days of the science of chemistry, there was no systematic
approach to naming compounds. Chemists coined names such as sugar of lead, quicklime, milk of magnesia, Epsom salts (see magnesium), and laughing gas to describe familiar
compounds. Such names are called common or trivial names. As chemistry
advanced, it became evident that, if common names were
used for all known compounds, which number in the millions, great confusion
would result. It clearly would be impossible to memorize trivial names for such
a large number of compounds. Therefore a systematic nomenclature (naming process) has been
developed. There are, however, certain familiar compounds that are always
referred to by their common names. The systematic names for H2O and
NH3, for example, are never used; these vital compounds are known
only as water and ammonia, respectively.
The simplest chemical compounds are binary
compounds—those consisting of two elements. Different rules apply for the
nomenclature of binary ionic compounds and binary molecular (covalent)
compounds, and so they will be considered separately.
The nomenclature for binary ionic compounds
simply entails naming the ions according to the following
rules:
A simple cation (obtained from a single atom)
takes its name from its parent element. For example, Li+
is called lithium in the names of compounds
containing this ion. Similarly, Na+ is called sodium, Mg2+ is called magnesium, and so on.
A simple anion (obtained from a single atom) is
named by taking the root of the parent element’s name and adding the suffix
-ide. Thus, the F− ion is called fluoride, Br− is called bromide, S2− is called sulfide, and so on.
The following examples illustrate the
nomenclature rules for binary ionic compounds:
compound
|
ions present
|
name
|
NaCl
|
Na+,
Cl−
|
|
KI
|
K+,
I−
|
|
CaS
|
Ca2+,
S2−
|
|
CsBr
|
Cs+,
Br−
|
|
MgO
|
Mg2−,
O2−
|
In the formulas of ionic compounds, simple ions
are represented by the chemical symbol for the element: Cl means Cl−,
Na means Na+, and so on. When individual ions are shown, however,
the charge is always included. Thus, the formula of potassium bromide is given as KBr, but, when
the potassium and bromide ions are shown individually, they are written K+
and Br−.
When a given metal atom can form more than one
type of cation, the charge on the particular cation present must be specified
in the name of the compound. For example, lead (Pb) can exist as Pb2+
or Pb4+ ions in ionic compounds. Also, iron (Fe) can form Fe2+
or Fe3+ ions, tin (Sn) can form Sn2+
or Sn4+ ions, gold (Au) can form Au+
or Au3+ ions, and so on. Therefore, the names of binary compounds
containing metals such as these must include a Roman numeral to specify the
charge on the ion. For example, the compound FeCl3, which contains
Fe3+, is named iron(III) chloride. On the other hand, the
compound FeCl2, which contains Fe2+, is designated as iron(II) chloride. In each case, the Roman
numeral in the name specifies the charge of the metal ion present.
Common simple
cations and anions
cation
|
name
|
anion
|
name
|
H+
|
hydrogen
|
H−
|
hydride
|
Li+
|
lithium
|
F−
|
fluoride
|
Na+
|
sodium
|
Cl−
|
chloride
|
K+
|
potassium
|
Br−
|
bromide
|
Cs+
|
cesium
|
I−
|
iodide
|
Be2+
|
beryllium
|
O2−
|
oxide
|
Mg2+
|
magnesium
|
S2−
|
sulfide
|
Ca2+
|
calcium
|
||
Ba2+
|
barium
|
||
Al3+
|
aluminum
|
||
Ag+
|
silver
|
||
An alternative system for naming compounds
containing metals that form only two ions is sometimes seen, especially in
older literature. The ion with the higher charge has a name ending in -ic, and
the one with the lower charge has the suffix -ous. For example, Fe3+
is called the ferric ion, and Fe2+ is called the ferrous ion. The
names for FeCl3 and FeCl2 are then ferric chloride and ferrous chloride, respectively.
Common ions that form multiple cations















Комментариев нет:
Отправить комментарий